Yinghui Feng, Yalong Cong, Yue Zhao, Chuanxi Zhang, Hucheng Song, Bohuan Fang,
Furong Yang, Huitu Zhang, John Z. H. Zhang* and Lujia Zhang*
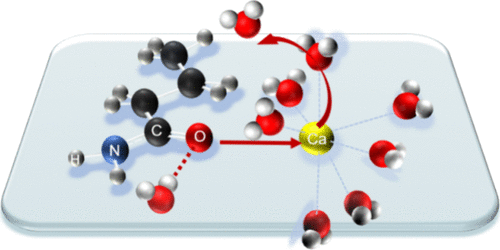
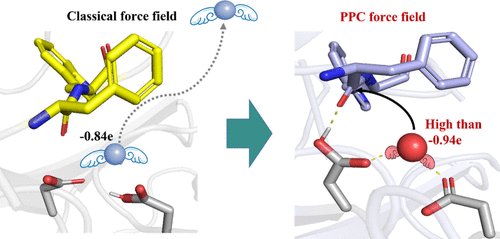
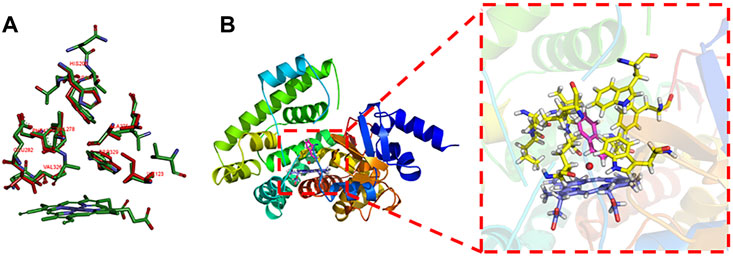
Hydrolysis catalyzed by aspartic proteases is a crucial reaction in many biological processes. However, anchoring water molecules and unifying multiple catalytic pathways remain significant challenges. Consequently, molecular design often compromises by focusing on enhancing substrate specificity. Using our self-developed polarizable point charge (PPC) force field, we determined the significant role of polarization in the hydrolase of pepsin for the first time. To be stably anchored in the active site, the water should be intensely polarized with a charge higher than −0.94e. Induced by this polarization, the pepsin was shown to support three general base/general acid pathways, with a preference for the gemdiol-intermediate-based pathway. Consequently, we proposed the “Blade of Polarized Water Molecule” model for rational enzyme design, highlighting that the polarization of both the attacking water and the attacked carbonyl is crucial for enhancing hydrolysis. Mutants D290Q and S172P showed activity enhancements of 191.23% and 324.70%, respectively. The improved polarization of water, carbonyl, and relevant nucleophilic attack distances in the mutants reaffirmed the crucial role of polarization in improving hydrolysis. This study provides a new perspective on hydrolase analysis and modification.