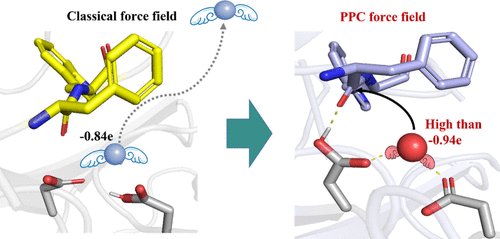
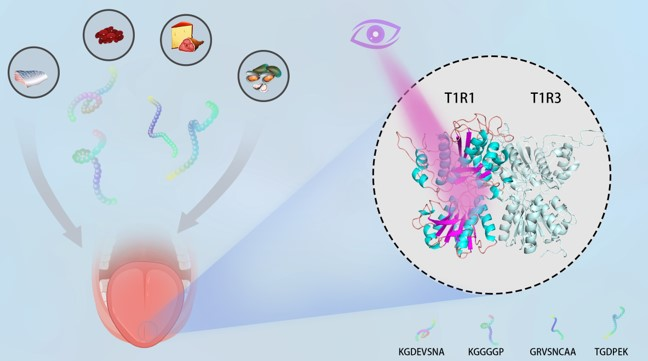
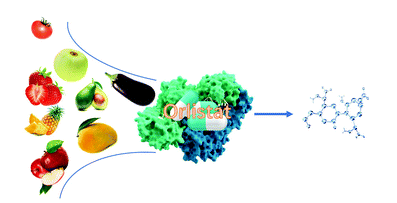
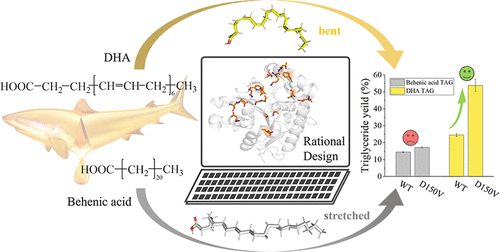
Yue Zhao, Yulu Miao, Yalong Cong, Jiawei Wang, Fengdong Zhi, Yue Pan, Jianguo Zhang, John Z.H. Zhang**, Lujia Zhang*
Pepsin, a typical aspartic protease, is extensively used to hydrolyze large proteins in the food industry. However, the lack of an industrial expression system and mutants with high activity hinders its industrial application. Here, we aimed to promote the industrial production of pepsin and improve pepsin activity to overcome these prob lems. The highest reported shake-flask yield of pepsin in Pichia pastoris was achieved by establishing a solid plate screening protocol. Based on the low sequence identity but the high structural similarity of aspartic proteases, a novel scissor-like model was proposed. This model revealed that pepsin activity was improved upon enhancing the stability of the conserved, connecting domain. Following this, a rational design strategy was employed, and potentially favorable mutants were screened. As a result, the activity of the L167F mutant significantly increased by 1172 U⋅mg 1, and structural analyses revealed enhanced internal stability of the conserved domain in the mutant. These results indicate that the stability of the connecting domain is an influential factor in aspartate protease activity. Furthermore, our findings exemplify the rational design of distal residues in the enzyme structure.