Yinghui Feng,Xin Yan,Mingzhe Ma,Ruyi Chen,Chuanxi Zhang,Yalong Cong,Bohuan Fang,Chunchi Chen,Longhai Dai,Hao Li,Haiming Jiang,Hong Sun,Hao Wei,Reyting Guo,Bei Gao,John Z.H.Zhang,and Lujia Zhang
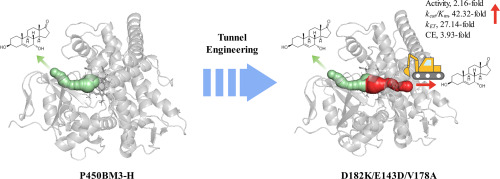
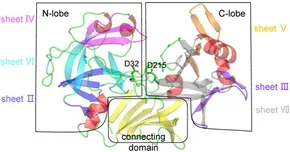
One-step amine–carboxyl dehydration condensation in cells (100% aqueous phase) is the most efficient and sustainable natural method for peptide and protein synthesis. However, most peptide ligases need modifications of substrates at the C- or N-terminal. To create this ligase, we engineered a “water-shielded” reaction chamber in protease subtilisin-P225A through precise polarization calculation using our self-developed PPC force field, thereby converting the hydrolysis reaction to a ligation reaction. We marked the first success and achieved 12 monomutants at first-round mutagenesis. The combined mutant P225A/N62L/S63L/Y217L/N218F with the highest activity was named Aqualigase. The X-ray structural and HDX-MS analysis confirmed a 20%–50% reduction in proton exchange and 50% elimination of water from the active site, demonstrating the success of the “water-shielding effect”. With Aqualigase/N158E, we successfully achieved the one-step synthesis of teriparatide, addressing the long-standing challenges in long-chain peptide or protein ligation. Notably, Aqualigase was also able to catalyze dealcoholizing ligation, transamidation, and esterification reactions. Its suitability for the length, size, and even the N- or C-terminal sequence composition of peptides or protein provides a huge scope for in situ protein conjunction in cells and peptide synthesis in industry.